Interested in learning more about how REZZAYO® could help in your hospital?
Request an appointment with a Napp representative
REZZAYO®: Demonstrated activity against a broad range of Candida species1–3
Mycological response was demonstrated for a range of Candida species, including:1 C. albicans C. glabrata C. krusei C. tropicalis C. parapsilosis
Mycological eradication at day 14 in patients with C. glabrata infection
In a pooled analysis in patients with C. glabrata infection only, at Day 14 REZZAYO® had a mycological eradication of 32/38 (1 - supplementary appendix). In the caspofungin group mycological response was 22/35. (treatment difference 21.4%, 95% CI 1.9-40.8)1
This analysis was not powered to infer differences between patients treated with REZZAYO® and caspofungin.2 Interpret with caution, not controlled for inferential statistics.
*ReSTORE (Phase III) and STRIVE (Phase II) were international, multicentre, double-blind, randomised controlled trials conducted in adults with invasive candidiasis and/or candidaemia. The studies were similarly designed allowing for a pre-planned analysis of the pooled data from patients receiving similar study treatments which included 294 adult patients meeting inclusion criteria of having systemic signs of invasive candidiasis and/or candidaemia plus mycological evidence obtained from blood or a normally sterile sampling site within 96 hours before randomisation. The primary efficacy outcome of global response at day 14 could not be pooled due to difference in definitions between the two studies. The primary pooled efficacy outcome was all-cause mortality at day 30, secondary pooled efficacy outcomes included mycological eradication at day 5 and 14. Time to first negative blood culture was an exploratory pooled efficacy outcome.2
Learn more about the pooled study design in this summary
Mycological response at day 5, according to baseline Candida species in the pooled analysis (mITT population)
This analysis was not powered to address differences between treatments.1 Interpret with caution, not controlled for inferential statistics.
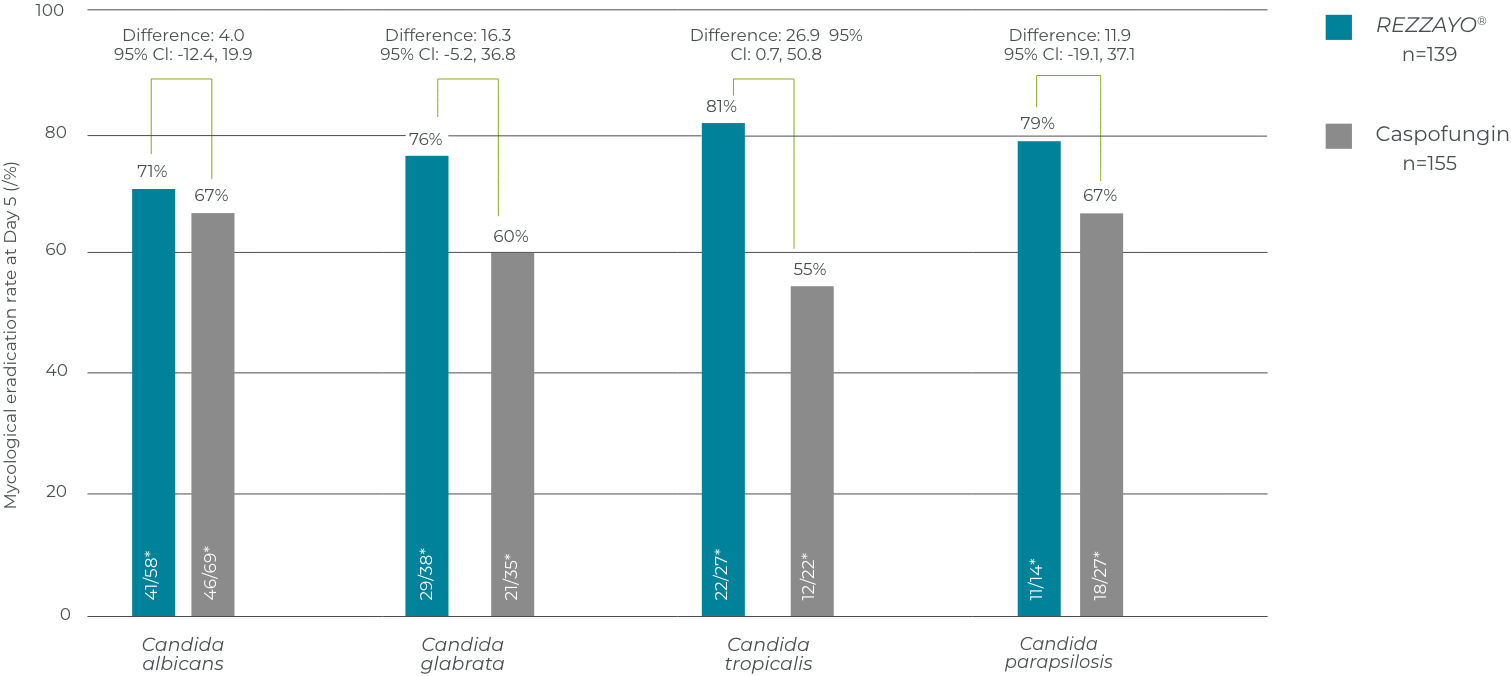
Adapted from Thompson GR III, et al. 2023.2
Mycological response at day 14, according to baseline Candida species in the pooled analysis (mITT population)
This analysis was not powered to address differences between treatments.1 Interpret with caution, not controlled for inferential statistics.
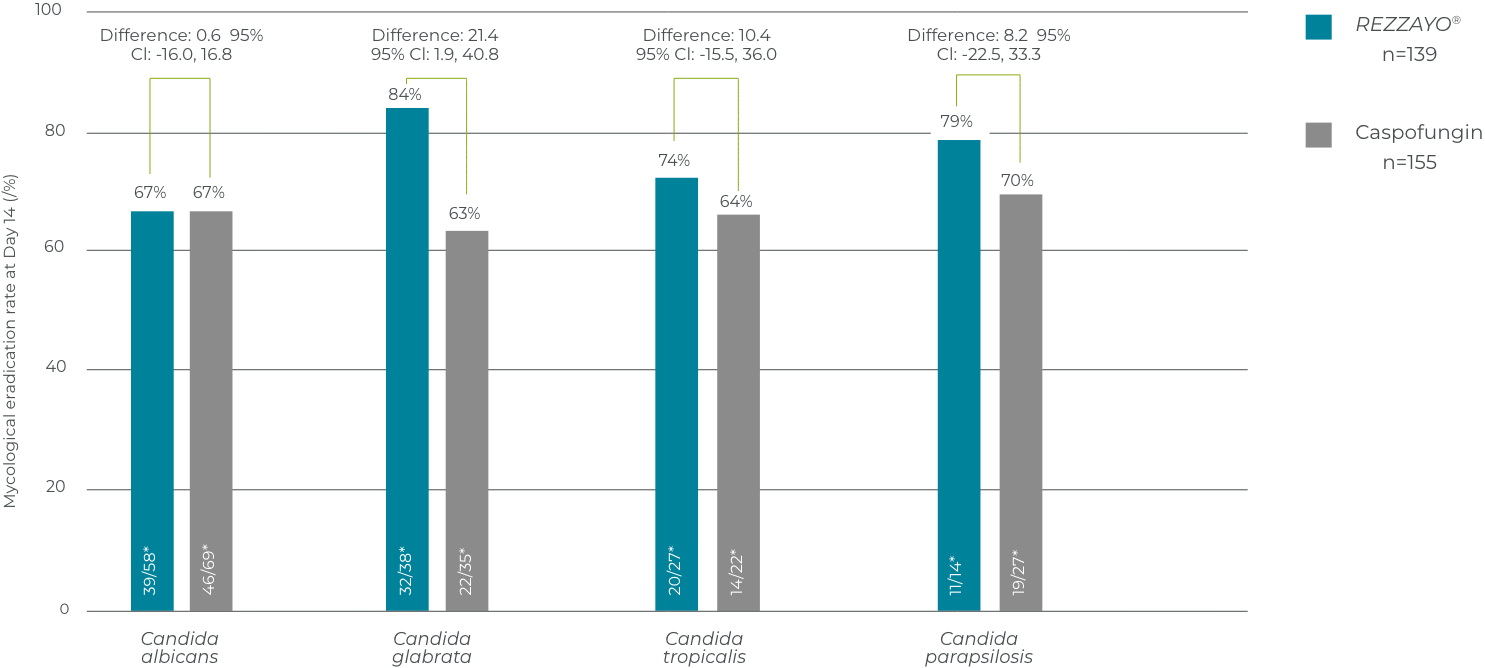
Adapted from Thompson GR III. et al 2023 Supplementary appendix 12
*n/N = number of subjects with Candida species demonstrating mycological eradication/total number of subjects with the corresponding species at baseline. mITT population includes all subjects who had documented Candida infection based on Central Laboratory evaluation of a culture from blood or another normally sterile site obtained ≤4 days (96 hours) before randomisation and received ≥1 dose of study drug.
In vitro activity against a broad range of clinically relevant Candida species, including:
Mycological response was shown for a range of Candida species, including: C. albicans C. glabrata C. krusei C. tropicalis C. parapsilosis
Adapted from Thompson GR III. et al 2023 Supplementary appendix 12
*In vitro activity against a group of C. auris isolates.3
Efficacy of REZZAYO® in treating infections caused by these isolates has not been established in clinical trials. In vitro data does not imply clinical efficacy.
mITT, modified intent-to-treat.
- REZZAYO® (rezafungin). Summary of Product Characteristics 2024.
- Thompson GR III, et al. Lancet Infect Dis. 2023; Pooled Analysis. Supplementary appendix 1 doi: 10.1016/S1473-3099(23)00551-0.
- Fioriti S, et al. J Fungi (Basel). 2022;8(10):1077.
- Govrins M and Lass-Flörl C. Nat Rev Microbiol. 2023 Sep 6. doi: 10.1038/s41579-023-00961-8.
- Zhao Y, et al. Cell Microbiol. 2016;18:1308–1316.
- Berkow EL and Lockhart SR. Diagn Microbiol Infect Dis. 2018;90:196–197.
Adverse events should be reported. Reporting forms and information can be found at http://yellowcard.mhra.gov.uk/.
Adverse events should also be reported to Napp Pharmaceuticals Limited on 01223 424444 or drugsafetyUKandROI@mundipharma.com.
®: REZZAYO is a Registered Trademark of Napp Pharmaceutical Group Limited.










