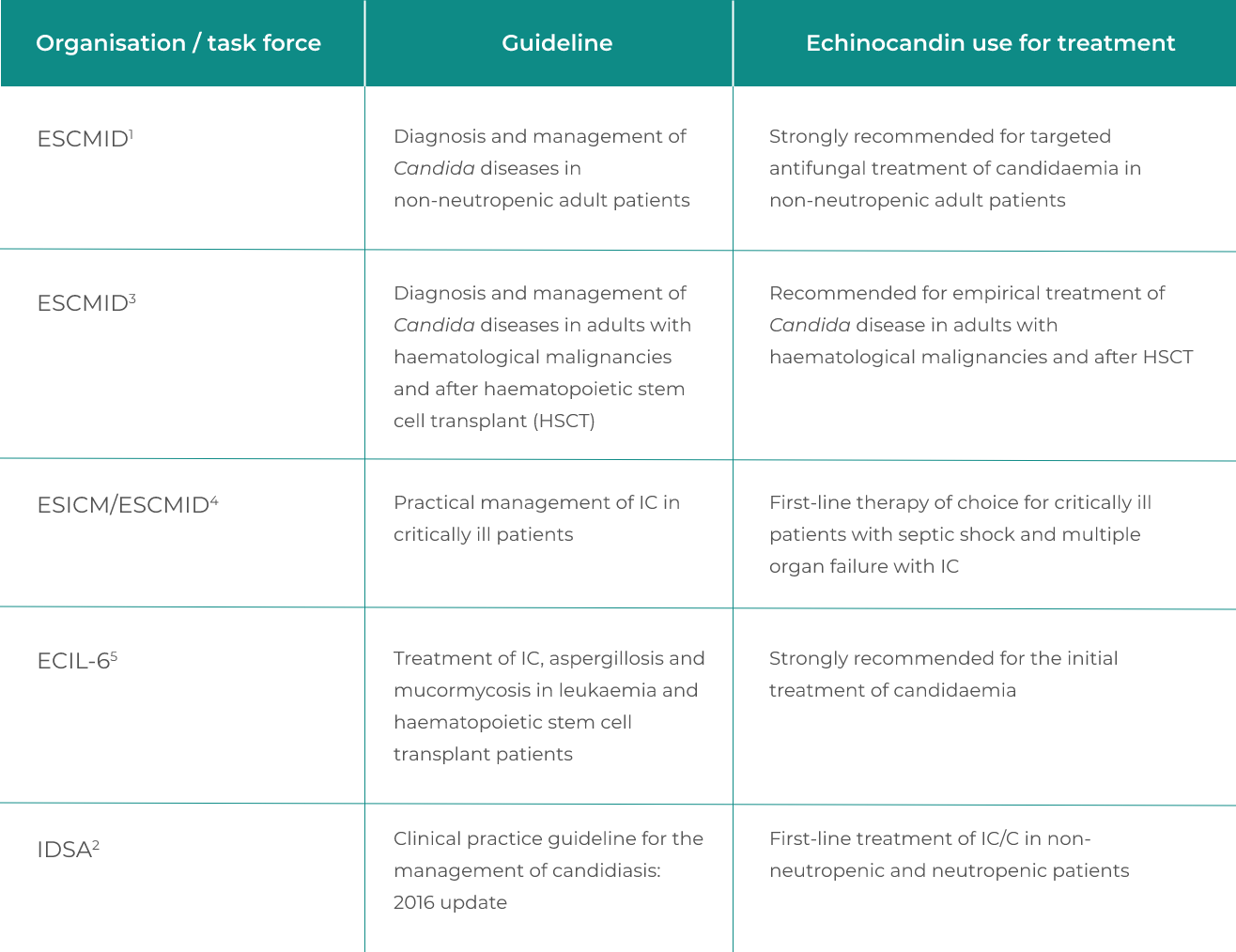
International guidelines recommend echinocandins as first-line treatment for invasive candidiasis1-5
Current European and international guidelines recommend the use of echinocandins for:
- Initial and empirical treatment of invasive candidiasis in non-neutropenic and neutropenic patient groups1-5
- The strength of recommendation is the same (strongly recommended) for anidulafungin, caspofungin and micafungin and is also the same for the overall and the haematologic populations5 (although the quality of evidence is lower for haematologic patients compared to the overall population as the number of neutropenic patients recruited in the clinical trials was low)
Summary of guideline recommendations for the use of echinocandins
Learn more about the management of invasive candidiasis
ECIL, European Conference on Infections in Leukemia; ESICM, European Society of Intensive Care Medicine; ESCMID, European Society of Clinical Microbiology and Infectious Diseases; HSCT, haematopoietic stem cell transplant; IDSA, Infectious Diseases Society of America.
- Cornely OA, et al. Clin Microbiol infect. 2012;18(Suppl 7):19–37.
- Pappas PG, et al. Clin Infect Dis. 2016;62(4):e1–e50.
- Ullmann AJ, et al. Clin Microbiol Infect. 2012;18(Suppl 7):53–67.
- Martin-Loeches I, et al. Intensive Care Med. 2019;45(6):789–805.
- Tissot F, et al. Haematologica. 2017;102(3):433–444.
Adverse events should be reported. Reporting forms and information can be found at http://yellowcard.mhra.gov.uk/.
Adverse events should also be reported to Napp Pharmaceuticals Limited on 01223 424444 or drugsafetyUKandROI@mundipharma.com.









